Cyclopentadithiophene-benzoic acid copolymers as conductive binders for silicon nanoparticles in anode electrodes of lithium ion batteries†
Abstract
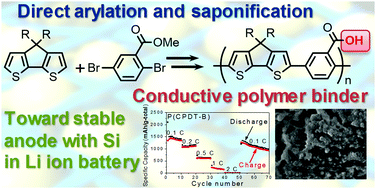
Cyclopentadithiophene and methyl-2,5-dibromobenzoate have been copolymerised via palladium complex catalysed direct arylation. The methyl ester group in the benzoate unit is converted to the carboxyl group via saponification. The polymers are mixed with Si nanoparticles for use as conducting binders in the fabrication of an anode electrode in lithium ion batteries. The battery with the electrode incorporating the saponified polymer shows much higher specific capacity of up to 1820 mA h g−1 (total weight) and a higher stability compared with the battery including the polymer before the saponification.


 Please wait while we load your content...
Please wait while we load your content...