Copper-catalyzed/mediated borylation reactions of epoxides with diboron reagents: access to β-hydroxyl boronic esters†
Abstract
We report the first copper-catalyzed/mediated borylative ring opening reaction of epoxides. This process represents a direct borylative C(sp3)–O bond cleavage of terminal epoxide substrates with commercially available diboron reagents. A wide range of epoxides with different functional groups are involved, and were subsequently converted to the corresponding β-hydroxyl boronic esters smoothly. Moreover, the ring opening product β-pinacol boronate alcohol provided a more beneficial approach for the formation of C–C and C–N bonds.
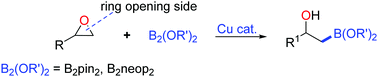


 Please wait while we load your content...
Please wait while we load your content...