The synergistic effect between KLVFF and self-assembly chaperones on both disaggregation of beta-amyloid fibrils and reducing consequent toxicity†
Abstract
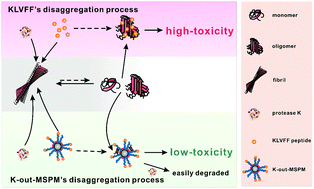
By combining KLVFF peptide and self-assembly chaperone we fabricate a new system to achieve the synchronization between Aβ fibril disaggregation and reducing toxicity of Aβ fragments (monomers or oligomers) that consequently formed. When the KLVFF peptides disaggregate fibrils into fragments, the hydrophobic domains of self-assembly chaperones promptly bind them at the same time. This binding blocks the re-aggregation of the fragments and their interaction with cells, and hence reduces the toxicity of these dangerous fragments.
- This article is part of the themed collection: Celebrating the ChemComm Emerging Investigator Lectureship Winners

 Please wait while we load your content...
Please wait while we load your content...