Green and highly efficient synthesis of perylene and naphthalene bisimides in nothing but water†
Abstract
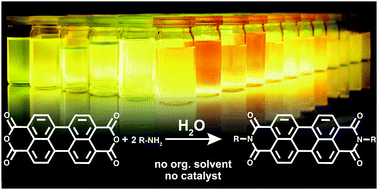
High-purity, symmetrically substituted perylene and naphthalene bisimides were obtained by hydrothermal condensation of monoamines with the corresponding bisanhydride. The hydrothermal imidization proceeds quantitatively, without the need for organic solvents, catalysts or excess of the amines.
- This article is part of the themed collection: Most downloaded articles of 2017: Organic and Biological Chemistry

 Please wait while we load your content...
Please wait while we load your content...