Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells†
Abstract
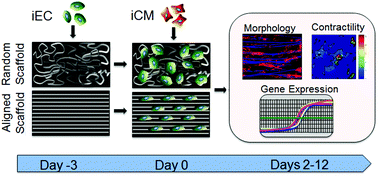
Engineering of myocardial tissue constructs is a promising approach for treatment of coronary heart disease. To engineer myocardial tissues that better mimic the highly ordered physiological arrangement and function of native cardiomyocytes, we generated electrospun microfibrous polycaprolactone scaffolds with either randomly oriented (14 μm fiber diameter) or parallel-aligned (7 μm fiber diameter) microfiber arrangement and co-seeded the scaffolds with human induced pluripotent stem cell-derived cardiomyocytes (iCMs) and endothelial cells (iECs) for up to 12 days after iCM seeding. Here we demonstrated that aligned microfibrous scaffolds induced iCM alignment along the direction of the aligned microfibers after 2 days of iCM seeding, as well as promoted greater iCM maturation by increasing the sarcomeric length and gene expression of myosin heavy chain adult isoform (MYH7), in comparison to randomly oriented scaffolds. Furthermore, the benefit of scaffold anisotropy was evident in the significantly higher maximum contraction velocity of iCMs on the aligned scaffolds, compared to randomly oriented scaffolds, at 12 days of culture. Co-seeding of iCMs with iECs led to reduced contractility, compared to when iCMs were seeded alone. These findings demonstrate a dominant role of scaffold anisotropy in engineering cardiovascular tissues that maintain iCM organization and contractile function.
- This article is part of the themed collection: Emerging Investigators 2017


 Please wait while we load your content...
Please wait while we load your content...