Abstract
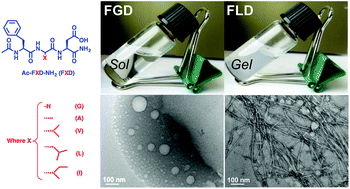
Supramolecular chemistry enables the creation of a diversity of nanostructures and materials. Many of these have been explored for applications as biomaterials and therapeutics. Among them, self-assembling peptides have been broadly applied. The structural diversity afforded from the library of amino acid building blocks has enabled control of emergent properties across length-scales. Here, we report on a family of amphiphilic tripeptides with sequence-controlled nanostructure. By altering one amino acid in these peptides, we can produce a diversity of nanostructures with different aspect-ratio and geometry. Peptides that produce high aspect-ratio structures can physically entangle to form hydrogels, which support cell viability in culture. Importantly, in comparison to many other short self-assembling peptide biomaterials, those reported here form filamentous nanostructures in the absence of typical secondary structures (i.e., β-sheet). Thus, we have illustrated a facile way to obtain versatile biomaterials with different nanostructural morphology from short and defined peptide sequences.
- This article is part of the themed collection: Emerging Investigators 2017


 Please wait while we load your content...
Please wait while we load your content...