Chemometric approaches to low-content quantification (LCQ) in solid-state mixtures using Raman mapping spectroscopy†
Abstract
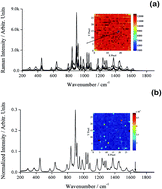
The low-content quantification (LCQ) of active pharmaceutical ingredients or impurities in solid mixtures is important in pharmaceutical manufacturing and analysis. We previously demonstrated the feasibility of using Raman mapping of the micro-scale heterogeneity in solid-state samples combined with partial least squares (PLS) regression for LCQ in a binary system. However, PLS is limited by the need for relatively large calibration sample numbers to attain high accuracy, and a rather significant computational time requirement for processing large Raman maps. Here we evaluated alternative chemometric methods which might overcome these issues. The methods were: net analyte signal coupled with classical least squares (NAS-CLS), multivariate curve resolution (MCR), principal component analysis with CLS (PCA-CLS), and the ratio of characteristic analyte/matrix bands combined with shape-preserving piecewise cubic polynomial interpolation curve fitting (BR-PCHIP). For high (>1.0%) piracetam analyte content, all methods were accurate with relative errors of prediction (REP) of <1.1%. For LCQ (0.05–1.0% w/w), three methods were able to predict piracetam content with reasonable levels of accuracy: 6.97% (PCA-CLS), 9.13% (MCR), and 12.8% (NAS-CLS). MCR offered the best potential as a semi-quantitative screening method as it was ∼40% quicker than PLS, but was less accurate due to being more sensitive to spectral noise factors.


 Please wait while we load your content...
Please wait while we load your content...