Convenient fluorescence detection of Cr(iii) in aqueous solution based on the gold nanoparticle mediated release of the acridine orange probe†
Abstract
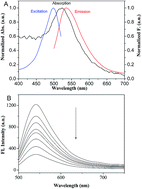
A simple and very sensitive method for detecting Cr3+ ions was developed. The method was based on fluorescence resonance energy transfer between acridine orange (AO) and gold nanoparticles (AuNPs), with AO acting as the donor and the AuNPs acting as acceptors. The method takes advantage of the high quenching efficiency of AuNPs and the large absorption band shift from 520 to 650 nm when AuNPs aggregate. The high quenching efficiency is caused by the non-covalent binding of AO to a AuNP surface through electrostatic interactions, forming an AO/AuNP system. Adding Cr3+ ions causes the intense fluorescence of the AO to be recovered because the Cr3+ ions induce the AuNPs to aggregate and the AO to be released from the AuNP surfaces. Under optimal conditions, the change in fluorescence intensity when Cr3+ ions were added was proportional to the Cr3+ concentration over the range of 0–12 μM. The detection limit was 0.02 μM. The system offers a new quantitative method for determining Cr3+ ions.


 Please wait while we load your content...
Please wait while we load your content...