Reaction screening and optimization of continuous-flow atropine synthesis by preparative electrospray mass spectrometry†
Abstract
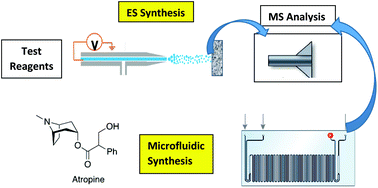
Preparative electrospray (ES) exploits the acceleration of reactions in charged microdroplets to perform a small scale chemical synthesis. In combination with on-line mass spectrometric (MS) analysis, it constitutes a rapid screening tool to select reagents to generate specific products. A successful reaction in preparative ES triggers a refined microfluidic reaction screening procedure which includes the optimization for stoichiometry, temperature and residence time. We apply this combined approach for refining a flow synthesis of atropine. A successful preparative ES pathway for the synthesis of the phenylacetyl ester intermediate, using tropine/HCl/phenylacetyl chloride, was optimized for solvent in both the preparative ES and microfluidics flow systems and a base screening was conducted by both methods to increase atropine yield, increase percentage conversion and reduce byproducts. In preparative ES, the first step yielded 55% conversion (judged using MS) to intermediate and the second step yielded 47% conversion to atropine. When combined in two discrete steps in continuous-flow microfluidics, a 44% conversion of the starting material and a 30% actual yield of atropine were achieved. When the reactions were continuously telescoped in a new form of preparative reactive extractive electrospray (EES), atropine was synthesized with a 24% conversion. The corresponding continuous-flow microfluidics experiment gave a 55% conversion with an average of 34% yield in 8 min residence time. This is the first in depth study to utilize telescoped preparative ES and the first use of dual ESI emitters for multistep synthesis.


 Please wait while we load your content...
Please wait while we load your content...