Fast electrochromic display: tetrathiafulvalene–graphene nanoflake as facilitating materials†
Abstract
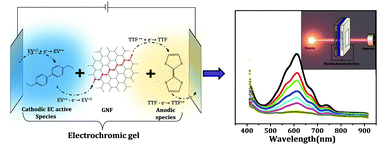
A new electrochromic gel (EC-Gel)-based active material has been prepared by using ethyl viologen (EV)–graphene nanoflakes (GNFs)–tetrathiafulvalene (TTF) for a faster and more efficient electrochromism. A prototype flexible electrochromic device has been fabricated by using the above-mentioned EC-Gel as an active layer which shows overall improved coloring efficiency as high as 208 (C cm−2)−1. At the same time, the abovementioned electrochromism shows color switching at a bias of 1.6 V with coloration/bleaching times as low as 0.4 and 0.9 seconds, respectively, which is better in comparison to that of other traditional EC-Gel or non EC-Gel-based electrochromic devices. Redox activity of EV–TTF pair results in such a fast bias induced color switching. Besides acting as an electrolyte, GNFs also facilitate achieving a faster bleaching time by allowing reversing the redox process quickly. The abovementioned facilitation is done by temporarily storing the electrons, which are released by EV during coloring cycle, to be supplied to TTF in the bleaching cycle through ballistic channels in graphene. An in situ UV-Vis spectroscopy establishes a transmission change of ∼45% in its stable state reversibly for more than 2500 cycles when the device is tested under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...