(Z)-Tetraphenylbut-2-ene-1,4-diones: facile synthesis, tunable aggregation-induced emission and fluorescence acid sensing†
Abstract
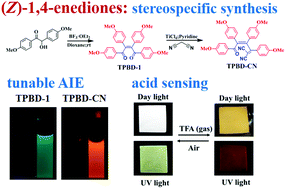
A facile approach to synthesize stereospecific (Z)-aryl-functionalized 1,4-enediones has been presented. The resulting molecules (TPBD-1 and TPBD-2) were demonstrated as a new type of heteroatom-containing AIE-active luminogens with multiple sites for structural modifications. Knoevenagel reaction of TPBD-1 led to a high electron affinity cyano derivative (TPBD-CN), which showed enhanced AIE performance and pronounced red-emission. More interestingly, TPBD-1 exhibited acid-induced red emission both in dilute solution and in the solid state, which is attractive due to its great potential for turn-on optical sensing. The reversible fluorescence acid sensing can be attributed to the stepwise binding effect of carbonyl groups on the 1,4-enedione core to rigidify the molecular conformation and strengthen the D–A interaction, which was systematically investigated by UV-vis, FL, FT-IR, and NMR spectroscopy and was further corroborated by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...