Compositional control of precipitate precursors for lithium-ion battery active materials: role of solution equilibrium and precipitation rate†
Abstract
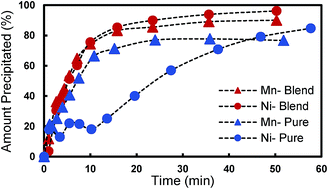
Multicomponent transition metal oxides are among the most successful lithium-ion battery cathode materials, and many previous reports have described the sensitivity of final electrochemical performance of the active materials to the detailed composition and processing. Coprecipitation of a precursor template is a popular, scalable route to synthesize these transition metal oxide cathode materials because of the homogeneous mixing of the transition metals within the particles, and the morphology control provided by the precursors. However, the deviation of the precursor composition from feed conditions is a challenge that has generally not been reported in previous studies. Using a target final material of the high voltage spinel LiMn1.5Ni0.5O4 as an example, we show in this study that the compositional deviation caused by coprecipitation can be significant under certain conditions, impacting the calcined final material structure and electrochemical properties. The study herein provides insights into the role of solution equilibrium and rate of precipitation of the transition metals during precipitate formation on precursor, and thus final active material, composition. Such knowledge is necessary to rationally predict and tune multicomponent battery precursor compositions synthesized via coprecipitation with high levels of accuracy.


 Please wait while we load your content...
Please wait while we load your content...