One-pot synthesis of silica–titania binary nanoparticles with acid–base pairs via biomimetic mineralization to fabricate highly proton-conductive membranes†
Abstract
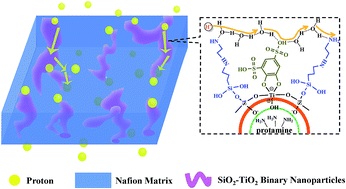
Simultaneous manipulation of vehicle-type and Grotthuss-type proton conduction within proton exchange membranes (PEMs) to induce satisfactory proton conductivity is crucial and challenging for promising environmentally friendly devices such as PEM fuel cells. In this study, a facile one-pot biomimetic mineralization approach is proposed for the construction of binary SiO2–TiO2 nanoparticles with a tunable ratio of silica to titania. Then the nanoparticles are functionalized by acid–base pairs and introduced into a Nafion matrix to fabricate novel hybrid membranes. The interaction between functional groups on SiO2–TiO2 binary nanoparticles and the polymer endows the hybrid membrane with good interfacial compatibility and enhanced dimensional stability. The incorporation of acid–base pairs reduces the activation energy for proton transfer; as a result, the hybrid membrane exhibits the highest proton conductivity of 1.37 × 10−2 S cm−1 at 26.1% RH and 80 °C, which is two orders of magnitude higher than that of recast Nafion. Compared with recast Nafion, a 51.3% increase in maximum power density is achieved for the Nafion/Si1–Ti2-160 hybrid membrane at 60 °C.


 Please wait while we load your content...
Please wait while we load your content...