Rapid microwave-assisted synthesis of hybrid zeolitic–imidazolate frameworks with mixed metals and mixed linkers†
Abstract
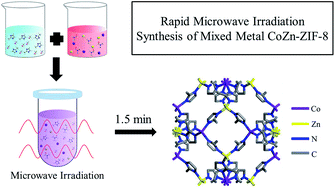
Herein we report a new microwave-assisted synthetic strategy to rapidly prepare hybrid zeolitic–imidazolate frameworks (ZIFs): ZIFs with mixed metal centers and/or mixed linkers. The microwave-based method significantly shortens synthesis time, produces a higher yield, substantially reduces the amounts of ligands, and eliminates the use of deprotonating agents. The X-ray diffraction pattern reveals that mixed metal CoZn–ZIF-8 (i.e., ZIF-8 with both Co and Zn centers) maintains the sodalite (SOD) zeolitic topology from the ZIF-8 parent. Elemental mapping using energy-dispersive X-ray spectroscopy (EDS) and electronic/geometric information obtained from X-ray absorption spectroscopy (XAS) confirm the uniform distribution of tetrahedral Co and Zn metal centers within the same framework of the mixed-metal ZIF. The metal to nitrogen (M–N) stretching frequencies of IR bands were observed to be systematically blue-shifted as the Co/Zn ratio in the mixed metal ZIF increases. Furthermore, for the first time, a hybrid ZIF with both mixed metal centers (Co and Zn) and mixed linkers (2-methylimidazolate and benzimidazolate) was prepared through one-step microwave synthesis. Finally, mixed metal CoZn–ZIF-8 with a Co/Zn ratio of ∼1 was grown as membranes on porous α-Al2O3 supports, showing a higher propylene/propane separation factor (∼120) when compared to pure Zn–ZIF-8 membranes (∼63) prepared by a similar method.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...