Electrochemical properties and structural evolution of O3-type layered sodium mixed transition metal oxides with trivalent nickel†
Abstract
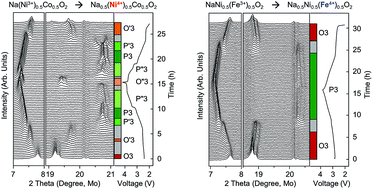
The electrochemical properties of NaNi0.5Co0.5O2 and NaNi0.5Fe0.5O2 and their structural transitions as a function of Na extraction associated with redox reactions are investigated in this work. Synthesized in the O3-type layered structure, both materials show reasonable electrochemical activities at room temperature, delivering approximately 0.5 Na per formula unit at C/10 discharge. More Na can be reversibly cycled in NaNi0.5Co0.5O2 at elevated temperature and/or in an extended voltage window, while NaNi0.5Fe0.5O2 shows significant capacity fading at a high voltage cutoff which is likely due to Fe4+ migration. In situ X-ray diffraction shows that the structural changes in the two materials upon desodiation are very different. NaNi0.5Co0.5O2 goes through many different two-phase reactions including three different O3-type and three different P3-type structures during cycling, producing a voltage profile with multiple plateau-like features. In contrast, NaNi0.5Fe0.5O2 has a smooth voltage profile and shows the typical O3–P3 phase transition without lattice distortion seen in other materials. This different structural evolution upon desodiation and re-sodiation can be explained by the electronic structure of the mixed transition metals and how it perturbs the ordering between Na ions differently.


 Please wait while we load your content...
Please wait while we load your content...