Preparation of –SO3−-coated nanopromoters for methane hydrate formation: effects of the existence pattern of –SO3− groups on the promotion efficiency†
Abstract
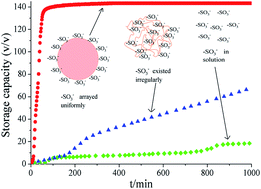
Sodium dodecyl sulfate (SDS) has been confirmed to be the most efficient promoter of gas hydrate formation; however, the foam generation during hydrate dissociation severely limits its application. In this study, the –SO3− group, similar to the hydrophilic group of SDS, was covalently fixed on polystyrene nanoparticles to prepare –SO3−-coated nanopromoters (–SO3−@PSNS) for methane hydrate formation. The existing form of –SO3− groups was controlled by varying the ratio of the hydrophobic and hydrophilic monomers during emulsion polymerization, which produced significant influence on the promotion efficiency. At the initial pressure of 5 MPa, when –SO3− groups existed irregularly with the macromolecules of –SO3−@PSNS in solution (–SO3−@PSNS-1), the growth rate was merely 8.02 ± 0.95 × 10−6 mol min−1 mL−1; however, when –SO3− groups were uniformly arrayed on the surface of the –SO3−@PSNS nanospheres (–SO3−@PSNS-2-3-4), the growth rate reached 18.08 ± 3.29–40.97 ± 2.89 × 10−6 mol min−1 mL−1. When nanopromoters with regularly arrayed –SO3− groups (–SO3−@PSNS-2-3) were used at the initial pressure of 6 MPa, the entire hydrate formation process was completed within 1–2 h and the methane storage capacity reached 142 and 137 v/v, indicating much better promotion compared to other common promoters, such as SDS, nanofluids, and activated carbon. Moreover, –SO3−@PSNS-3 resulted in no foam generation during hydrate dissociation and produced excellent recycling performance in 8 cycles of methane hydrate formation. Therefore, the –SO3−-coated nanopromoters developed in this study have significant potential in the industrial application of hydrate-based natural gas storage and transportation.


 Please wait while we load your content...
Please wait while we load your content...