Performance evaluation of gasoline alternatives using a thermodynamic spark-ignition engine model†
Abstract
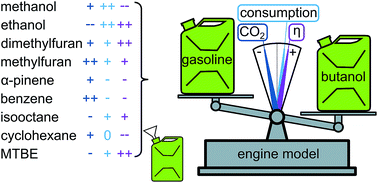
In light of climate change and due to the fact that surface transportation heavily relies on internal combustion engines, many different alternatives to gasoline have been proposed. Herein, we present a model, incorporating only first order effects, which allows a quick assessment of the suitability of a certain molecule as a replacement for gasoline. Using global sensitivity analysis, the elemental composition and the vapor heat capacity have been identified as main influencing fuel parameters. A case study using the currently proposed alternative fuels (methanol, ethanol, n-butanol, dimethylfuran, methylfuran, and α-pinene) as well as gasoline and several hydrocarbons (cyclohexane, n-heptane, isooctane, and benzene) revealed n-butanol as the best performing alternative fuel. The use of this compound entails a significant decrease in CO2 emissions and an increased efficiency, but also a higher consumption in comparison with gasoline.


 Please wait while we load your content...
Please wait while we load your content...