Au(iii)-aryl intermediates in oxidant-free C–N and C–O cross-coupling catalysis†
Abstract
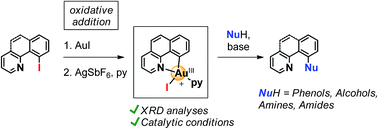
Au(III)-aryl species have been unequivocally identified as reactive intermediates in oxidant-free C–O and C–N cross coupling catalysis. The crystal structures of cyclometalated neutral and cationic Au(III) species are described and their key role in 2 electron-redox Au(I)/Au(III) catalysis in C–O and C–N cross couplings is shown. Nucleophiles compatible with Au-catalyzed cross couplings include aromatic and aliphatic alcohols and amines, as well as water and amides.


 Please wait while we load your content...
Please wait while we load your content...