Elucidating the mechanism of the Ley–Griffith (TPAP) alcohol oxidation†
Abstract
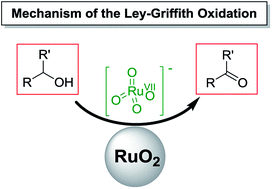
The Ley–Griffith reaction is utilized extensively in the selective oxidation of alcohols to aldehydes or ketones. The central catalyst is commercially available tetra-n-propylammonium perruthenate (TPAP, n-Pr4N[RuO4]) which is used in combination with the co-oxidant N-methylmorpholine N-oxide (NMO). Although this reaction has been employed for more than 30 years, the mechanism remains unknown. Herein we report a comprehensive study of the oxidation of diphenylmethanol using the Ley–Griffith reagents to show that the rate determining step involves a single alcohol molecule, which is oxidised by a single perruthenate anion; NMO does not appear in rate law. A key finding of this study is that when pure n-Pr4N[RuO4] is employed in anhydrous solvent, alcohol oxidation initially proceeds very slowly. After this induction period, water produced by alcohol oxidation leads to partial formation of insoluble RuO2, which dramatically accelerates catalysis via a heterogeneous process. This is particularly relevant in a synthetic context where catalyst degradation is usually problematic. In this case a small amount of n-Pr4N[RuO4] must decompose to RuO2 to facilitate catalysis.


 Please wait while we load your content...
Please wait while we load your content...