Acid- and Au(i)-mediated synthesis of hexathymidine-DNA-heterocycle chimeras, an efficient entry to DNA-encoded libraries inspired by drug structures†
Abstract
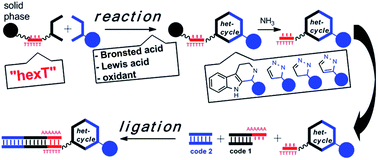
Libraries of DNA-tagged compounds are a validated screening technology for drug discovery. They are synthesized through combinatorial iterations of alternated coding and preparative synthesis steps. Thus, large chemical space can be accessed for target-based screening. However, the need to preserve the functionality of the DNA tag severely restricts the choice of chemical methods for library synthesis. Acidic organocatalysts, transition metals, and oxidants furnish diverse drug-like structures from simple starting materials, but cause loss of genetic information by depurination. A hexathymidine oligonucleotide, called “hexT” allows the chemist utilizing these classes of catalysts to access a potentially broad variety of structures in the initial step of library synthesis. We exploited its catalyst tolerance to efficiently synthesize diverse substituted β-carbolines, pyrazolines, and pyrazoles from readily available starting materials as hexT conjugates by acid- and Au(I)-catalysis, respectively. The hexT conjugates were ligated to coding DNA sequences yielding encoded screening libraries inspired by drug structures.


 Please wait while we load your content...
Please wait while we load your content...