Synthesis of bioactive and stabilized cyclic peptides by macrocyclization using C(sp3)–H activation†
Abstract
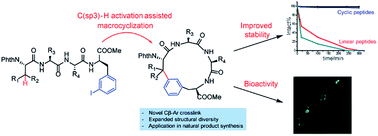
Cyclic peptides have attracted increasing attention in recent years due to their ability to inhibit protein–protein interactions. Current strategies to prepare cyclic peptides often rely on functional amino acid side chains or the incorporation of unnatural amino acids, thus limiting their structural diversity. Here, we describe the development of a highly versatile peptide macrocyclization strategy through a palladium-catalyzed C(sp3)–H activation and the synthesis of cyclic peptides featuring unique hydrocarbon linkages between the β-carbon of amino acids and the aromatic side chains of Phe and Trp. We demonstrate that such peptides exhibit improved biological properties compared to their acyclic counterparts. Finally, we applied this method in the synthesis of the natural product celogentin C.
- This article is part of the themed collection: Celebrating a century of chemical excellence at Nanjing University


 Please wait while we load your content...
Please wait while we load your content...