Selective uni- and bidirectional homologation of diborylmethane†
Abstract
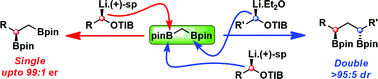
Diborylmethane can be homologated uni- and bidirectionally by using enantiomerically pure lithium-stabilized carbenoids to give 1,2- and 1,3-bis(boronic esters), respectively, in good yield and with excellent levels of enantio- and diastereoselectivity. The high sensitivity of the transformation to steric hindrance enables the exclusive operation of either manifold, effected through the judicious choice of the type of carbenoid, which can be a sparteine-ligated or a diamine-free lithiated benzoate/carbamate. The scope of the 1,2-bis(boronic esters) so generated is complementary to that encompassed by the asymmetric diboration of alkenes, in that primary–secondary and primary–tertiary 1,2-bis(boronic esters) can be prepared with equally high levels of selectivity and that functional groups, such as terminal alkynes and alkenes, are tolerated. Methods for forming C2-symmetric and non-symmetrical anti and syn 1,3-bis(boronic esters) are also described and represent a powerful route towards 1,3-functionalized synthetic intermediates.


 Please wait while we load your content...
Please wait while we load your content...