Selective N-terminal functionalization of native peptides and proteins†
Abstract
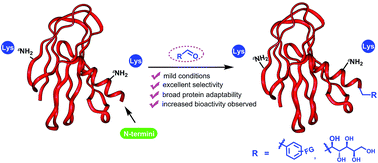
We report an efficient, highly selective modification on the N-terminal amines of peptides and proteins using aldehyde derivatives via reductive alkylation. After modification of a library of unprotected peptides XYSKEASAL (X varies over 20 natural amino acids) by benzaldehyde at room temperature, pH 6.1 resulted in excellent N-terminal selectivity (α-amino/ε-amino: >99 : 1) and high reaction conversion for 19 out of the 20 peptides. Under similar conditions, highly selective N-terminal modifications were achieved with a variety of aldehydes. Furthermore, N-termini of native peptides and proteins could be selectively modified under the same conditions to introduce bioorthogonal functional groups. Using human insulin as an example, we further demonstrated that preserving the positive charge in the N-terminus using reductive alkylation instead of acylation leads to a 5-fold increase in bioactivity. In summary, our reported method provides a universal strategy for site-selective N-terminal functionalization in native peptides and proteins.


 Please wait while we load your content...
Please wait while we load your content...