Coevolution and ratiometric behaviour in metal cation-driven dynamic covalent systems†
Abstract
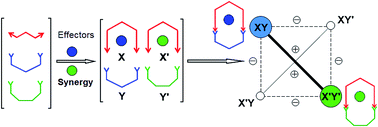
Dynamic Covalent Libraries (DCLs) have been used to demonstrate coevolution behaviour on a molecular level using dynamic covalent molecules such as imines and hydrazones. Two systems are presented: the first system is based on a dialdehyde and two diamines in combination with Zn(II) and Hg(II) to form a 2 × 2 Constitutional Dynamic Network (CDN) of four complexes of macrocyclic bis-imines. Whereas the two metal ions, when reacted separately form a complex with each macrocycle with low selectivity, when applied together, each cation yields selectively a complex with one of the two macrocycles. Thus, the simultaneous application of both cations, where one might have expected the formation of four different complexes, results in the synergistic evolution (co-evolution) towards a simpler, more selective outcome under agonist amplification. The second system of 4 components, 2 amines and 2 aldehydes displays metalloselection together with a correlated evolution in distribution on complexation of Zn(II) and Cu(I) with the dynamic ligand constituents and exhibits a dynamic ratiometry process related to the antagonistic behaviour of a pair of ligand constituents.


 Please wait while we load your content...
Please wait while we load your content...