Carbon dioxide binding at a Ni/Fe center: synthesis and characterization of Ni(η1-CO2-κC) and Ni-μ-CO2-κC:κ2O,O′-Fe†
Abstract
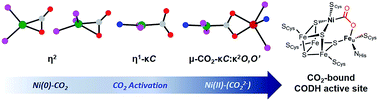
The degree of CO2 activation can be tuned by incorporating a distinct electronic coordination environment at the nickel center. A mononuclear nickel carboxylate species (Ni–CO2, 3) and a dinuclear nickel–iron carboxylate species (Ni–CO2–Fe, 5) were prepared. The structure of 3 reveals a rare η1-κC binding mode of CO2, while that of 5 shows bridging CO2 binding (μ2-κC:κ2O,O′) between the nickel and iron, presented as the first example of a nickel-μ-CO2-iron species. The structural analyses of 3 and 5 based on XRD and DFT data reveal a higher degree of CO2 activation in 5, imparted by the additional interaction with an iron ion.

 Please wait while we load your content...
Please wait while we load your content...