Photoredox radical conjugate addition of dithiane-2-carboxylate promoted by an iridium(iii) phenyl-tetrazole complex: a formal radical methylation of Michael acceptors†
Abstract
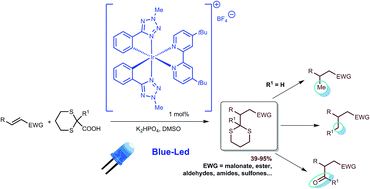
A readily accessible iridium(III) phenyl-tetrazole complex ([Ir(ptrz)2(tBu-bpy)]+, 2; Hptrz = 2-methyl-5-phenyl-tetrazole; tBu-bpy = 4,4′-di-tert-butyl-2,2′-bipyridine) is shown to be a versatile catalyst for a new photocatalytic Michael reaction. Under light irradiation in the presence of 2, a dithiane 2-carboxylic acid, obtained by simple hydrolysis of a commercially available ethyl ester, generates a 1,3-dithiane radical capable of performing addition to a variety of Michael acceptors (e.g., unsaturated ketones, esters, amides and malonates). This broad scope reaction with high yields is a formal photo-redox addition of the elusive methyl radical and the adducts obtained can be starting materials for a variety of functionalized products. The excited-state oxidation potential of catalyst 2 allows selective formation of radicals only from α-heterosubstituted carboxylates. Chemical modification of this metal complex can tune the electrochemical properties, opening a route to new highly selective catalytic photo-oxidation reactions.


 Please wait while we load your content...
Please wait while we load your content...