Mild and selective base-free C–H arylation of heteroarenes: experiment and computation†
Abstract
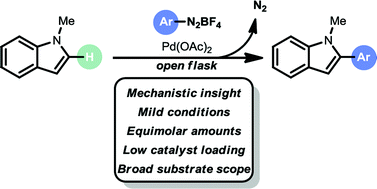
A mild and selective C–H arylation strategy for indoles, benzofurans and benzothiophenes is described. The arylation method engages aryldiazonium salts as arylating reagents in equimolar amounts. The protocol is operationally simple, base free, moisture tolerant and air tolerant. It utilizes low palladium loadings (0.5 to 2.0 mol% Pd), short reaction times, green solvents (EtOAc/2-MeTHF or MeOH) and is carried out at room temperature, providing a broad substrate scope (47 examples) and excellent selectivity (C-2 arylation for indoles and benzofurans, C-3 arylation for benzothiophenes). Mechanistic experiments and DFT calculations support a Heck–Matsuda type coupling mechanism.
- This article is part of the themed collection: Celebrating our 2025 Prizewinners


 Please wait while we load your content...
Please wait while we load your content...