Tuning reaction products by constrained optimisation†
Abstract
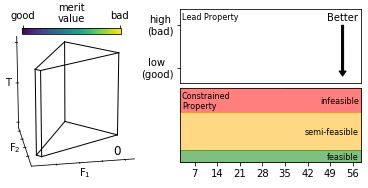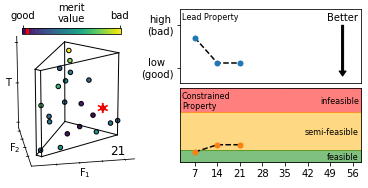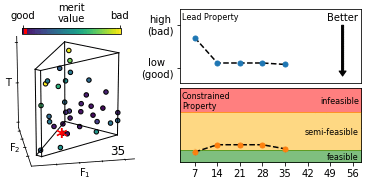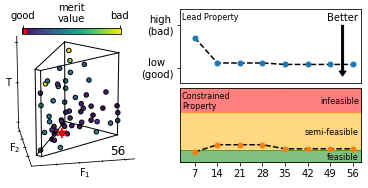
We describe an effective means of defining optimisation criteria for self-optimising reactors, applicable to situations where a compromise is sought between several competing objectives. The problem is framed as a constrained optimisation, in which a lead property is optimised subject to constraints on the values that other properties may assume. Compared to conventional methods (using weighted-sum- and weighted-product-based merit functions), the approach described here is more intuitive, easier to implement, and yields an optimised solution that more faithfully reflects user preferences. The method is applied here to the synthesis of o-xylenyl adducts of Buckminsterfullerene, using a cascadic reaction of the form X0 → X1 → X2 → … XN. Specifically, we selectively target the formation of the (technologically useful) first- and second-order adducts X1 and X2, while at the same time suppressing the formation of unwanted higher-order products. More generally, the approach is applicable to any chemical optimisation involving a trade-off between competing criteria. To assist with implementation we provide a self-contained software package for carrying out constrained optimisation, together with detailed tutorial-style instructions.


 Please wait while we load your content...
Please wait while we load your content...