Highlighting diffusional coupling effects in zeolite catalyzed reactions by combining the Maxwell–Stefan and Langmuir–Hinshelwood formulations†
Abstract
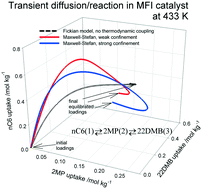
The combined phenomena of intra-crystalline adsorption, diffusion and reversible chemical reactions inside microporous crystalline zeolite catalyst particles are described by combining the Langmuir–Hinshelwood kinetics with the Maxwell–Stefan (M–S) diffusion formulation. Simulations of transient diffusion and reaction inside catalyst crystallites are performed for a variety of reactions including: alkane isomerization, xylene isomerization, ethylation of benzene and dehydrogenation of ethane. For all reaction systems, the transient diffusion/reaction process exhibits overshoots in the loading of the more mobile guest molecules within the zeolite pores; such overshoots imply the attainment of supra-equilibrium conversions for a limited time span. The origin of the transient overshoots is traceable to the use of chemical potential gradients as the proper driving forces in the M–S formulation; this leads to coupling effects induced by mixture adsorption thermodynamics. Use of the simplified Fick's law for intra-crystalline diffusion, ignoring thermodynamic coupling, does not result in overshoots. Simulations of fixed zeolite bed reactors are performed to demonstrate the significant differences in the productivity predicted by the M–S and Fick models for intra-crystalline diffusion.


 Please wait while we load your content...
Please wait while we load your content...