Fast synthesis of amides from ethyl salicylate under microwave radiation in a solvent-free system†
Abstract
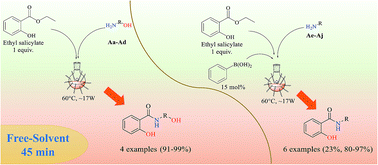
In this study, amide bond formation, one of the most important reactions in organic chemistry, it was evaluated using ethyl salicylate and ten different primary amines. Under the optimized experimental conditions, i.e. 60 °C, hexane, phenylboronic acid-PBA (15 mol%), boric acid-BA (15 mol%) or without catalyst-WC, using a hot-plate for 24 h, amides were obtained in excellent isolated yields (WC, 77–94% to S-Aa-Ad; PBA, 11–94% to S-Ae-Aj; and BA, 28–90% to S-Ae-Aj). The reaction employing CAL-B also permitted a moderate conversion for the production of amides S-Ae-Aj (3–42%). However, in our efforts to reduce the amide synthesis time (24 h), the reactions were performed in the presence of microwave-MW radiation using a free-solvent system [60 °C, PBA (15 mol%) or WC], which reduced the time of the reaction by 32-fold (45 min) and afforded nine amides (S-Aa-Ah and S-Aj) in 80–99% isolated yield and S-Ai in 23% yield. A cytotoxicity assay demonstrated that the amide S-Ag was capable of inhibiting four human tumor cell lines (MCF-7, HCT116, HepG2, and HL-60) with an IC50 ranging from 8.68 to 17.57 μg mL−1. In this study, MW radiation provided an attractive way for faster reactions, improved yields, and cleaner reactions, as well as the synthesis of amide S-Ag with cytotoxic activity.


 Please wait while we load your content...
Please wait while we load your content...