First total synthesis of the only known 2-isopropyliden-2H-benzofuran-3-one isolated from V. luetzelburgii†
Abstract
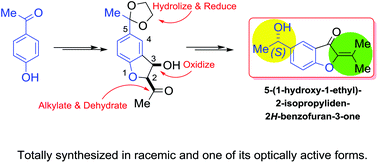
The first total synthesis of 5-(1-hydroxy-1-ethyl)-2-isopropyliden-2H-benzofuran-3-one, is reported in its racemic and in one of its optically active [(S)-(−)] forms. This heterocycle, isolated from Verbesina luetzelburgii, is the only known 2-isopropyliden-2H-benzofuran-3-one produced by species of Verbesina. The sequence took place in eight steps and 19% overall yield (up to 33% for the chiral form), from 4′-hydroxyacetophenone. It entailed carbonyl group protection as the 1,3-dioxolane and a phenol ortho formylation, followed by a Williamson etherification with chloroacetone and an organocatalytic cross-aldolization, to afford a 2-acetyl-2,3-dihydrobenzofuran-3-ol intermediate. The latter underwent a methyl Grignard addition to the carbonyl moiety, followed by selective oxidation of the benzylic alcohol and deprotection, resulting in a β-hydroxy diketone derivative. A MsCl-assisted dehydration of the tertiary alcohol, established the isopropylidene motif, whereas the syntheses culminated by chemical or enzymatic (carrot, celeriac) selective reductions of the exocyclic carbonyl group.


 Please wait while we load your content...
Please wait while we load your content...