Ancistrolikokines E–H and related 5,8′-coupled naphthylisoquinoline alkaloids from the Congolese liana Ancistrocladus likoko with antiausterity activities against PANC-1 human pancreatic cancer cells†
Abstract
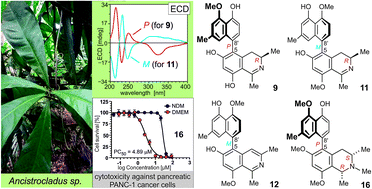
A striking feature of the metabolite profile of Ancistrocladus likoko (Ancistrocladaceae) is the exclusive production of 5,8′-linked naphthylisoquinoline alkaloids varying in their OMe/OH substitution patterns and in the hydrogenation degree in their isoquinoline portions. Here we present nine new compounds of this coupling type isolated from the twigs of this remarkable Central African liana. Three of them, the ancistrolikokines E (9), E2 (10), and F (11), are the first 5,8′-linked naphthyldihydroisoquinolines found in nature with R-configuration at C-3. The fourth new metabolite, ancistrolikokine G (12), is so far the only representative of the 5,8′-coupling type that belongs to the very rare group of alkaloids with a fully dehydrogenated isoquinoline portion. Moreover, five new N-methylated naphthyltetrahydroisoquinolines, named ancistrolikokines A2 (13), A3 (14), C2 (5), H (15), and H2 (16) are presented, along with six known 5,8′-linked alkaloids, previously identified in related African Ancistrocladus species, now found for the first time in A. likoko. The structural elucidation was achieved by spectroscopic analysis (HRESIMS, 1D and 2D NMR) and by chemical (oxidative degradation) and chiroptical (electronic circular dichroism) methods. The new ancistrolikokines showed moderate to good preferential cytotoxic activities towards pancreatic PANC-1 cells in nutrient-deprived medium (NDM), without causing toxicity under normal, nutrient-rich conditions, with ancistrolikokine H2 (16) being the most potent compound.


 Please wait while we load your content...
Please wait while we load your content...