Preparation, characterization and antitumor activity evaluation of silibinin nanoparticles for oral delivery through liquid antisolvent precipitation
Abstract
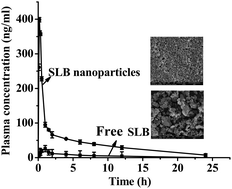
Silibinin (SLB) is reported to possess multiple biological activities. However, due to its poor water solubility and poor absorption after oral administration, its clinical therapeutic effects have been limited. Thus, an experiment is designed to prepare SLB nanoparticles by the liquid antisolvent precipitation (LAP) technique to improve its solubility and bioavailability. Firstly, we applied single-factor experiments to investigate the effects of various factors on the mean particle size (MPS) of SLB nanoparticles in the LAP process, and the optimal conditions obtained were: HPMC concentration 3 mg mL−1, precipitation temperature 55 °C, SLB concentration 35 mg mL−1, antisolvent/solvent volume ratio 10, dropping speed 1 mL min−1, stirring speed 800 rpm and stirring time 5 min. A SLB nanosuspension with a MPS of 132.3 nm was obtained under the optimum conditions. The SLB nanoparticles were obtained by the freeze-drying method, and characterized using various analytical techniques such as SEM, FTIR, XRD DSC, and TG. The experimental data revealed that SLB nanoparticles were transformed into an amorphous form without changing the chemical structure, and had a higher solubility, and were about 36.9 mg mL−1 (free SLB was about 0.09 mg mL−1) in artificial gastric juice (AGJ) and about 59.71 mg mL−1 (free SLB was about 0.03 mg mL−1) in artificial intestinal juice (AIJ). The dissolution rate of SLB nanoparticles was also obviously higher than that of free SLB, and was about 48.2 times and 153.8 times that of free SLB in AGJ and in AIJ. Furthermore, the results of a bioavailability study in rats showed that the Cmax value of SLB nanoparticles (398.580 ng mL−1) was apparently higher than that of free SLB (26.070 ng mL−1), and the AUC (0 → t) value of SLB nanoparticles (965.666 ng mL−1 h−1) was about 6.48 times greater than that of free SLB (149.124 ng mL−1 h−1), so the SLB nanoparticles had a higher bioavailability than free SLB. The inhibitory effect of SLB nanoparticles on HepG2 cells was also higher by lower IC50 than that of free SLB. Taken together, the present study suggests that the SLB nanoparticles can become a new oral drug formulation with high bioavailability and produce a better response for its clinical applications.


 Please wait while we load your content...
Please wait while we load your content...