A mechanistic study of ethanol transformation into ethene and acetaldehyde on an oxygenated Au-exchanged ZSM-5 zeolite†
Abstract
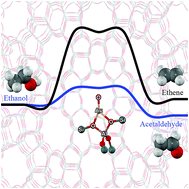
Ethanol transformation to ethene and acetaldehyde over low- and high-spin state oxygenated Au-exchanged ZSM-5 zeolite has been investigated using a well-validated density functional method, M06-L. The reaction initiates from the ethanol O–H bond dissociation leading to the formation the ethoxide–hydroxide intermediate with the activation energy of 9.5 kcal mol−1. This intermediate can be then decomposed to either ethene or acetaldehyde products. In the ethene production pathway, the decomposition of the ethoxide–hydroxide intermediate proceeds via the β-H–C scission with the activation energy of 40.5 kcal mol−1. For the acetaldehyde production pathway, the ethoxide–hydroxide intermediate transforms to acetaldehyde via α-H–C scission with the activation barrier of 10.6 kcal mol−1 which is significantly lower than the ethene pathway. The reaction rate for acetaldehyde formation is also found to be higher than the ethene one. The results suggest that the acetaldehyde product is thermodynamically and kinetically favored over ethene for the transformation of the ethanol on oxygenated Au-exchanged ZSM-5 zeolite.


 Please wait while we load your content...
Please wait while we load your content...