High-quality conjugated polymers via one-pot Suzuki–Miyaura homopolymerization†
Abstract
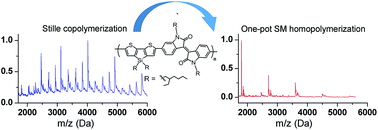
This paper describes a one-pot Suzuki–Miyaura homopolymerization that involves in situ borylation/cross coupling of dibrominated donor–acceptor conjugated macromonomers, in contrast to the standard Stille copolymerization of thienosilole and isoindigo monomers. Reaction kinetics investigation reveals that bis(pinacolato)diboron promotes an efficient polymerization. The homopolymer showed blue-shifted absorption compared to the Stille copolymer, which is rationalized by quantum chemical calculations of a series of oligomers containing various donor–acceptor configurations. The calculations suggest that the homopolymerization of asymmetrical macromonomers likely introduced both acceptor–acceptor and donor–donor segments into the backbone. The acceptor–acceptor segment is found to contribute mostly to the blue-shift of maximum absorption wavelength. Furthermore, detailed analysis of MALDI-TOF (matrix-assisted laser-desorption ionization-time of flight) spectra of these two polymers indicated that while the homopolymer is well defined, the Stille copolymer is end-capped mostly with the thienosilole moieties and/or methyl groups, implicating that destannylation and methyl transfer are the most-likely chain-termination pathways that limit high molecular weight. This is in sharp contrast to the homopolymerization, where chain-terminators are required to control the molecular weight for obtaining soluble material. The photovoltaic performances of bulk-heterojunction solar cells based on these polymers are compared.


 Please wait while we load your content...
Please wait while we load your content...