Intramolecular cyclization of N-allyl propiolamides: a facile synthetic route to highly substituted γ-lactams (a review)
Abstract
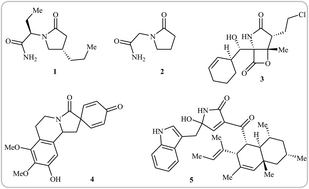
The development of simple and efficient methods for construction of substituted γ-lactams is an important synthetic goal because such ring skeletons are present in numerous natural compounds that display diverse biological activities. Intramolecular cyclization of N-allyl propiolamides is an efficient, economic, and operationally simple strategy for the synthesis of the titled compounds. In the present review we will discuss recent advances in the synthesis of functionalized γ-lactam derivatives from these easily accessible and versatile building blocks with the emphasis on the mechanistic aspects of the reactions.
- This article is part of the themed collection: 2017 Review articles


 Please wait while we load your content...
Please wait while we load your content...