Magnetically recyclable self-assembled thin films for highly efficient water evaporation by interfacial solar heating†
Abstract
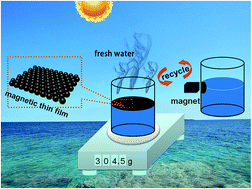
Magnetic microspheres including Fe3O4, MnFe2O4, ZnFe2O4, and CoFe2O4 have been synthesized via a simple solvothermal method followed by surface hydrophobization with 1H,1H,2H,2H-perfluorooctyltrichlorosilane. The hydrophobic magnetic microspheres can self-assemble into a thin film under simulated solar light irradiation and float on the surface of water. The formed film was used as photothermal material for water evaporation based on a new concept of interfacial solar heating. The water evaporation efficiency was significantly enhanced by the Fe3O4 thin film, and is about 1.4, 1.7 and 2.2 times higher than that without the formation of a Fe3O4 thin film, Fe3O4 uniformly dispersed in water, and water evaporation itself, respectively. The temperature gradient distributions from the surface to the bottom of the water directly demonstrated the advantage of interfacial solar heating for water evaporation. We believe that the water evaporation efficiency with the magnetic thin film is mainly due to the high light absorption, rapid heat transfer and good solid–liquid adhesion performance. In addition, the hydrophobic magnetic microspheres also have advantages over other reported photothermal materials due to their easy recycling, non-toxicity, low dose, and low cost.


 Please wait while we load your content...
Please wait while we load your content...