N-Propargylic β-enaminocarbonyls: powerful and versatile building blocks in organic synthesis
Abstract
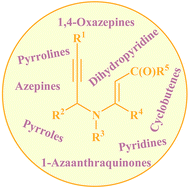
Nitrogen-containing heterocyclic compounds are not only prevalent in an extensive number of natural products and synthetic pharmaceuticals but are also used as building blocks in organic synthesis. The efficient preparation of highly functionalized N-heterocycles from cheap and easily available starting materials has therefore become of central interest for synthetic organic chemists. This review gives an overview of new developments in synthesis of highly substituted N-heterocycles, including pyrroles, pyridines, pyrrolines, piperidines, azepines, and related compounds, from N-propargylic β-enaminocarbonyls (N-propargylic β-enaminones and β-enaminoesters) in recent years. Mechanistic aspects of the reactions are considered and discussed in detail.
- This article is part of the themed collection: 2017 Review articles


 Please wait while we load your content...
Please wait while we load your content...