A red emitting of manganese-doped boron carbon oxynitride (BCNO) phosphor materials: facile approach and photoluminescence properties
Abstract
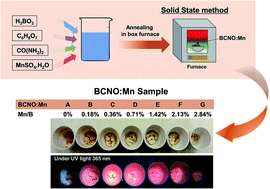
BCNO (boron carbon oxynitride) phosphors have attracted attention due to their non toxicity, simple synthesis process, and high quantum efficiency. However, the tunable emission, particularly a red emission, is still challenging to achieve. In the present study, a bright red emission (emission wavelength 620 nm under 365 nm UV excitation) of a manganese-doped BCNO (BCNO:Mn) phosphor synthesized by a solid state method is reported. Without Mn-doping, the BCNO phosphor exhibited a bright blue emission that can be ascribed to the closed-shell BO− and BO2− anions that act as luminescence center. Via Mn-doping, a red emission was exhibited that can be ascribed to the 4T1(4G) → 6A1(6S) transition from a new luminescence center of Mn2+ incorporated into the BCNO host lattice. The optimum red PL properties (λem: 611 nm, λex: 365 nm) were obtained with BCNO:Mn at a molar ratio of Mn/B at 0.71% mol/mol synthesized at 550 °C. We believe that the BCNO:Mn is a promising red-emitting phosphor for white light diodes.


 Please wait while we load your content...
Please wait while we load your content...