1,3-Dipolar cycloadditions with meso-tetraarylchlorins – site selectivity and mixed bisadducts†
Abstract
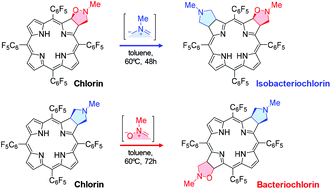
The 1,3-dipolar cycloaddition reaction of meso-tetraarylporphyrins with nitrones gives isoxazolidine-fused chlorins. Depending on the substitution pattern on the meso-aryl groups and the nitrone, the chlorins can be obtained in high yields (up to 91%). Bacteriochlorin-type bisadducts are also obtained, although in low yield, from the reaction of meso-tetrakis(pentafluorophenyl)porphyrin with N-methyl, N-cyclohexyl or N-benzyl nitrone. The structure of a bis(N-benzyl isoxazolidine-fused) bacteriochlorin was determined by single-crystal X-ray diffraction and rationalized by DFT calculations. To further explore the nature of site selectivity in the formation of bisadducts, isomeric mixed bacteriochlorins and isobacteriochlorins were synthesized by two complementary routes that involved the sequential addition of two types of 1,3-dipoles to the porphyrin macrocycle: a nitrone and an azomethine ylide. The photophysical properties of the mixed bacteriochlorins were evaluated and the results were compared with the reported data for other meso-tetraarylbacteriochlorins.


 Please wait while we load your content...
Please wait while we load your content...