Desulfurization–bromination: direct chain-end modification of RAFT polymers†
Abstract
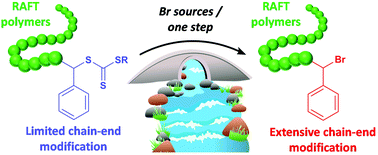
We report a simple and efficient transformation of thiol and thiocarbonylthio functional groups to bromides using stable and commercially available brominating reagents. This procedure allows for the quantitative conversion of a range of small molecule thiols (including primary, secondary and tertiary) to the corresponding bromides under mild conditions, as well as the facile chain-end modification of polystyrene (PS) homopolymers and block copolymers prepared by reversible addition–fragmentation chain transfer (RAFT) polymerization. Specifically, the direct chain-end bromination of PS prepared by RAFT was achieved, where the introduced terminal bromide remained active for subsequent modification or chain-extension using classical atom transfer radical polymerization (ATRP). This transformation sets the foundation for bridging RAFT and ATRP, two of the most widely used controlled radical polymerization (CRP) strategies, and enables the preparation of chain-end functionalized block copolymers not directly accessible using a single CRP technique.


 Please wait while we load your content...
Please wait while we load your content...