Singlet oxygen-mediated one-pot chemoselective peptide–peptide ligation†
Abstract
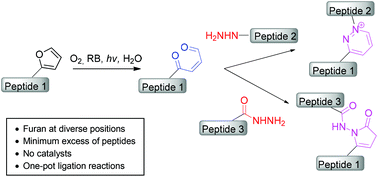
We here describe a furan oxidation based site-specific chemical ligation approach using unprotected peptide segments. This approach involves two steps: after photooxidation of a furan-containing peptide, ligation is achieved by reaction of the unmasked keto–enal with C- or N-terminal α-nucleophilic moieties of the second peptide such as hydrazine or hydrazide to form a pyridazinium or pyrrolidinone linkage respectively.
- This article is part of the themed collection: Celebrating Excellence in Research: 100 Women of Chemistry


 Please wait while we load your content...
Please wait while we load your content...