A bifunctional old yellow enzyme from Penicillium roqueforti is involved in ergot alkaloid biosynthesis†
Abstract
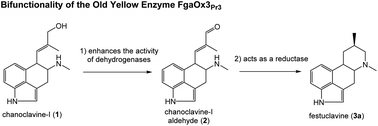
The blue cheese-making fungus Penicillium roqueforti produces isofumigaclavine A as the main ergot alkaloid. Recently, genome mining revealed the presence of two DNA loci bearing the genetic potential for its biosynthesis. In this study, a short-chain dehydrogenase/reductase (SDR) from one of the loci was proved to be responsible for the conversion of chanoclavine-I to its aldehyde. Furthermore, a putative gene coding for an enzyme with high homology to Old Yellow Enzymes (OYEs) involved in the ergot alkaloid biosynthesis was found outside the two clusters. Biochemical characterisation of this enzyme, named FgaOx3Pr3, showed that it can indeed catalyse the formation of festuclavine in the presence of a festuclavine synthase FgaFS, as had been observed for other OYEs in ergot alkaloid biosynthesis. Differing from other homologues, FgaOx3Pr3 does not convert chanoclavine-I aldehyde to its shunt products in the absence of FgaFS. Instead, it increases significantly the product yields of several SDRs for the conversion of chanoclavine-I to its aldehyde. Kinetic studies proved that overcoming the product inhibition is responsible for the observed enhancement. To the best of our knowledge, this is the first report on the bifunctionality of an OYE and its synergistic effect with SDRs.


 Please wait while we load your content...
Please wait while we load your content...