Selective synthesis of three product classes from imine and carboxylic acid precursors via direct imine acylation†‡
Abstract
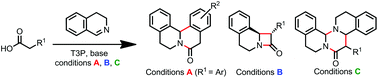
Three divergent Direct Imine Acylation (DIA) procedures are reported that allow the selective generation of δ-lactams, β-lactams and tetrahydropyrimidinones (via a novel three-component coupling) from imine and carboxylic acid precursors. All operate via initial N-acyliminium ion formation and diverge depending on the reaction conditions and nature of the substrates.


 Please wait while we load your content...
Please wait while we load your content...