Nazarov reaction: current trends and recent advances in the synthesis of natural compounds and their analogs
Abstract
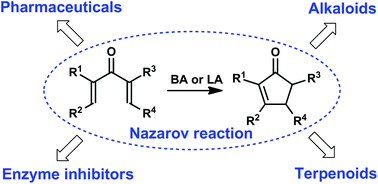
The Nazarov reaction (cyclization) is one of the most useful synthetic tools for the stereoselective preparation of various cyclopentenone scaffolds. This review summarizes recent applications of this reaction to the synthesis of natural compounds and their usefulness to pharmacology analogs published over 2005–2016. Modern protocols for the facile generation of key cationic intermediates and efficient catalytic, in particular asymmetric, versions of Nazarov cyclization are considered. Available literature data are for the first time classified according to the chemical structures of the products, which include relatively simple non-annulated functionalized cyclopentenoids and complex polynuclear cyclopentenone derivatives annulated with one or more non-aromatic, aromatic or heteroaromatic rings. This classification is convenient for specialists in organic synthesis and for researchers working in the area of medicinal and biomolecular chemistry.


 Please wait while we load your content...
Please wait while we load your content...