One-pot synthesis of 3,5-disubstituted 1,2,4-thiadiazoles from nitriles and thioamides via I2-mediated oxidative formation of an N–S bond†
Abstract
A simple and practical method for I2-mediated one-pot synthesis of 3-alkyl-5-aryl-1,2,4-thiadiazoles has been developed; the one-pot reaction includes sequential intermolecular addition of thioamides to nitriles, and intramolecular oxidative coupling of N–H and S–H bonds mediated by molecular iodine. Meanwhile the protocol uses readily available nitriles and thioamides as the starting materials, molecular iodine as the oxidant, and generates various 1,2,4-thiadiazoles in moderate to good yields with a wide array of functional groups. This method is an efficient approach for the synthesis of unsymmetrically disubstituted 1,2,4-thiadiazoles.
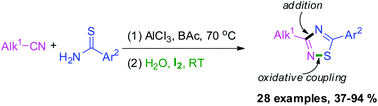


 Please wait while we load your content...
Please wait while we load your content...