A practical approach to asymmetric synthesis of dolastatin 10†
Abstract
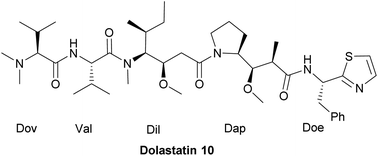
Dolastatin 10, an antineoplastic agent for cancer chemotherapy, is a linear peptide possessing N,N-dimethyl Val-OH, L-valine, (3R,4S,5S)-dolaisoleucine, (2R,3R,4S)-dolaproine and (S)-dolaphenine. Our efficient synthesis includes the following three key features: (1) SmI2-induced cross-coupling was employed to couple aldehyde 11 with (S)-N-tert-butanesulfinyl imine 12 to generate the required stereocenters of Dap (7); (2) asymmetric addition of chiral N-sulfinyl imine 10 provided a straightforward approach to the synthesis of the protected Doe ((S,S)-8); (3) a practical method to the key subunit Val-Dil (24a) has been established as an alternative synthetic route for the synthesis of this challenging chemical structure.


 Please wait while we load your content...
Please wait while we load your content...