Late-stage divergent synthesis and antifouling activity of geraniol–butenolide hybrid molecules†
Abstract
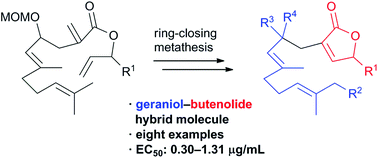
Hybrid molecules consisting of geraniol and butenolide were designed and synthesized by the late-stage divergent strategy. In the synthetic route, ring-closing metathesis was utilized for the construction of a butenolide moiety. A biological evaluation of the eight synthetic hybrid compounds revealed that these molecules exhibit antifouling activity against the cypris larvae of the barnacle Balanus (Amphibalanus) amphitrite with EC50 values of 0.30–1.31 μg mL−1. These results show that hybridization of the geraniol and butenolide structural motifs resulted in the enhancement of the antifouling activity.


 Please wait while we load your content...
Please wait while we load your content...