Synthesis of tetrafluoroethylene- and tetrafluoroethyl-containing azides and their 1,3-dipolar cycloaddition as synthetic application†
Abstract
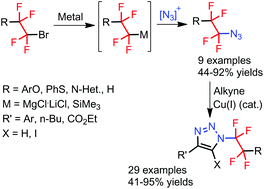
Tetrafluoroethylene-containing azides are accessed in two steps (one pot) from tetrafluoroalkyl bromides by metalation and reaction with electrophilic azides. Subsequent copper(I)-catalyzed azide–alkyne cycloaddition afforded N-tetrafluoroethyl and N-tetrafluoroethylene 4-substituted 1,2,3-triazoles. In addition, the protocol for the synthesis of 4,5-disubstituted 1,2,3-triazoles is presented.


 Please wait while we load your content...
Please wait while we load your content...