An unprecedented stereoselective base-induced trimerization of an α-bromovinylsulfone†
Abstract
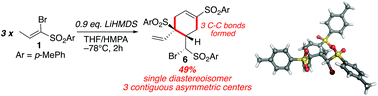
A unprecedented base-induced trimerization of bromovinylsulfone 1 afforded the cyclohexene 6 as a single diastereoisomer. Optimization of this reaction gave the adduct 6 in 49% yield. A mechanistic rationale for the trimerization involving consecutive SN2′ additions and [3,3]-rearrangements and a final stereoselective intramolecular conjugate addition is proposed and supported by M06-2X density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...